[Background] In order to solve the problems of energy shortage and environmental pollution in human society, large-scale grid storage and vehicle electrification based on renewable energy have attracted wide attention. Compared with lithium-ion battery, sodium-ion battery has a broader application prospect in large-scale power grid storage due to its advantages of abundant resources and low cost. The all-solid sodium battery based on solid electrolyte has the outstanding advantages of flame-retardant and explosion-proof, and can be directly matched with metal sodium negative electrode and high pressure positive electrode, which is expected to further improve the energy density and safety of sodium battery. Among them, Na3Zr2Si2PO12 (NZSP) solid electrolyte has attracted extensive research interest due to its advantages such as simple synthesis, high ionic conductivity at room temperature, wide electrochemical stability window and good chemical stability with sodium anode. Sodium at present, all solid state battery research still faces many challenges, including NZSP | | especially Na interface and its associated problems, such as: poor interface wettability and sodium anode utilization rate is low, it will cause a large interface resistance, sodium sodium dendrite growth and solid-state batteries than low energy problems. A great deal of work has been done to try to ameliorate the interface problem through surface impurity removal (e.g. heat treatment) and the introduction of sodiophilic interlayers (e.g. wetting media such as AlF3, SnS2, SiO2, and Sn/SnOx). However, compared with the heat treatment strategy, the introduction of the sodiophilic intermediate layer has a more significant effect on the improvement of sodium wetting, but the resistance of the passivation layer itself and the interface resistance between the passivation layer and the intermediate layer may still exist, and the interaction between the two layers is unclear. Therefore, efficient and practical NZSP | | Na cathode interface design strategy still needs to be developed, new strategy should take into account the removal of the surface passivation layer and the effective wetting of molten sodium, sodium and help improve the utilization rate of the cathode and the battery energy density.
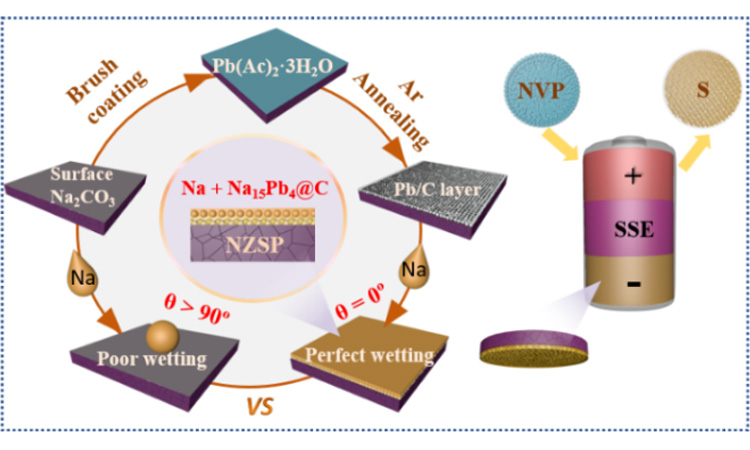
Abstract: The dual-function Pb/C intermediate layer is constructed to help realize low interfacial resistance, dendrite-free, high sodium utilization of solid state batteries
[Job Description] Recently, Professor Xiao Wen Zhan, Gao Shan, Lu Ke, Yuan Yupeng from Anhui University and Professor Xiao Di Ren from University of Science and Technology of China have introduced a simple and scalable Pb/C intermediate layer (Pb/C@NZSP) on the surface of NZSP solid electrolyte to solve the negative electrode interface and utilization problems. Pb/C @ NZSP surface at 120 ℃ can show perfect wettability of sodium (Na contact Angle of 0 tentacles, room temperature | | @ NZSP Pb/C interface resistance as low as 1.5 @ cm2; At 55℃, the sodium-symmetric battery achieved an ultra-long cycle life of 1800 h (0.5 mA cm-2/0.5 mAh cm-2), one of the best performances reported to date. In addition, we demonstrate in situ preparation of sodium negative electrode with controllable mass, and explore the effect of N/P ratio on the cycle performance of solid-state sodium batteries with Na3V2(PO4)3 and S as positive electrodes. When N/P=40.0, Na3V2(PO4)3 full battery can stable 300 cycles. In general, the Pb/C interlayer strategy introduced in this paper embodies the dual functions of stabilizing the negative sodium interface and improving the utilization rate of the negative sodium electrode, thus speeding up the practical application of all solid-state sodium batteries. The paper was published in the top international journal Chemical Science. Li Rui and Jiang Daochuan are co-first authors of this paper.
[Content description]
1. Construction of Pb/C intermediate layer
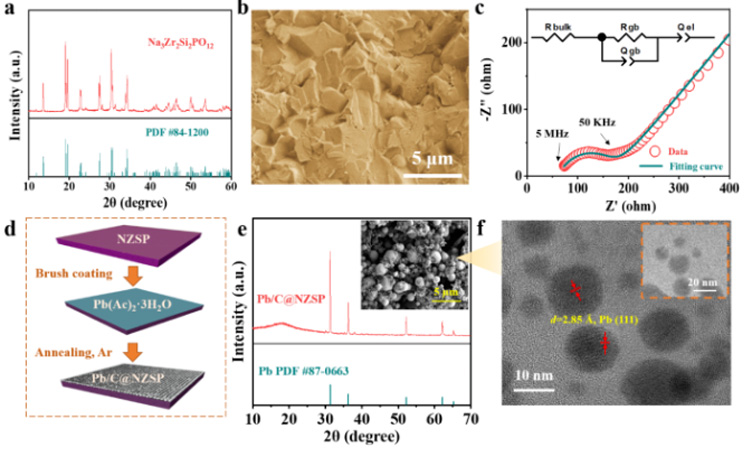
Figure 1. (a) XRD, (b) SEM and (c) impedance at room temperature of the NZSP solid electrolyte. (d) Schematic diagram of Pb/C interlayer construction. (e) XRD and SEM of Pb/C@NZSP. (f) TEM images of Pb/C samples scraped from Pb/C@NZSP surface.
2. Study on interface characteristics and mechanism
As shown in Figure 2a-b, Pb/C@NZSP electrolyte shows good sodium wetting characteristics (contact Angle: 0 solution). In contrast, fused sodium is almost non-wetting on unmodified NZSP tablets (contact Angle: 130.4°). Because of this, Pb/C @ NZSP electrolytes have close physical contact with Na, in Na | | NZSP interface can find obvious gap (FIG. 2 C - d). The composition of Pb/C@NZSP surface moistened by 10mg molten Na was analyzed by XRD. It was found that Na15Pb4 alloy was formed by reaction of partially molten Na and Pb. According to the theoretical model and DFT calculation, the adhesion work of Na(001)/Pb(001) is Wad= 0.44J m-2. Further combining with the Young-Dupre equation, the contact Angle which is more consistent with the experiment can be derived (Figure 2f-h, see the original for detailed discussion). It is worth noting that the untreated NZSP surface is closer to the situation shown in Figure 2g. XPS analysis also reveals the presence of sodium carbonate on the surface and proves that the Pb/C construction process effectively removes the function of the passivation layer on the NZSP surface (Figure 2i).
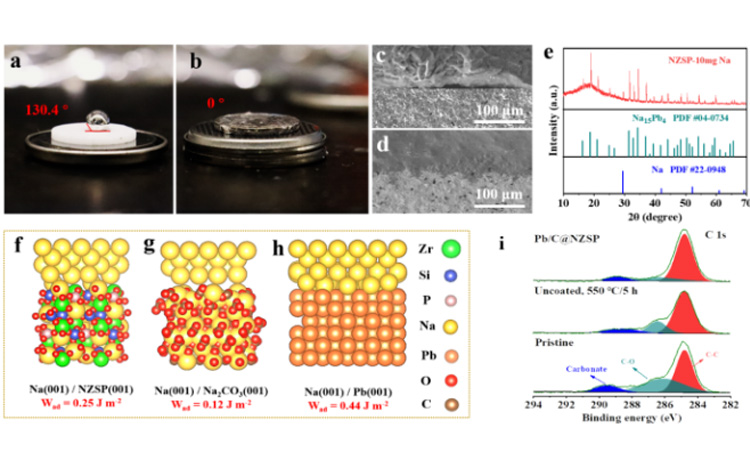
Figure 2. Contact Angle test of molten sodium on (a) NZSP and (b) Pb/C@NZSP surfaces at 120 ℃. (c) Na | | NZSP and (d) Na | | @ Pb/c NZSP cross section SEM figure. (e) XRD pattern of Pb/C@NZSP wetted with 10 mg sodium. Theoretical calculation model of (f) Na(001)/NZSP(001), (g) Na(001)/Na2CO3(001), (h) Na(001)/Pb(001) and adhesion work (Wad). (i) Untreated NZSP, C 1s XPS spectra of NZSP and Pb/C@NZSP heated at 550 ℃ in Ar for 5 h.
3. Symmetric battery test
Na | | NZSP | | Na symmetrical cells showed a large interface resistance (about 391 Ω cm2). The additional stacking pressure improves the interface physical contact. For example, at an optimal pressure of 15 MPa, the area specific resistance (ASRint) can be reduced to 87.5 to prevent interface 2 (Figure 3a). And thanks to the perfect wettability, preparation of molten sodium perfusion Na | | @ NZSP Pb/C interface, in the absence of additional stack pressure ASRint achieved under the condition of very low 1.5 Ω cm2 (FIG. 3 b), is one of the best record in the literature. The limiting current density (CCD) is defined as the lowest current density when a battery is short-circuited. Na | | @ NZSP Pb/C | | Na symmetric battery at all test temperatures of CCD were significantly higher than that of Na | | NZSP | | Na symmetric batteries, and in 70 when the battery is 1.4 cm 2, mA is almost Na | | NZSP | | Na mA (0.8 cm to 2) twice (figure 3 C). 55 ℃, Na | | @ NZSP Pb/C | | Na symmetric cell showed 1800 h of super long cycle life (0.5 mA mAh cm - 2 cm - 2/0.5, overpotential: 60 mV). Compared with the reported NZSP modification strategies, the sodium-symmetric cells using Pb/C@NZSP electrolytes have more outstanding overall performance (cyclic stability and interfacial resistance, FIG. 3e).
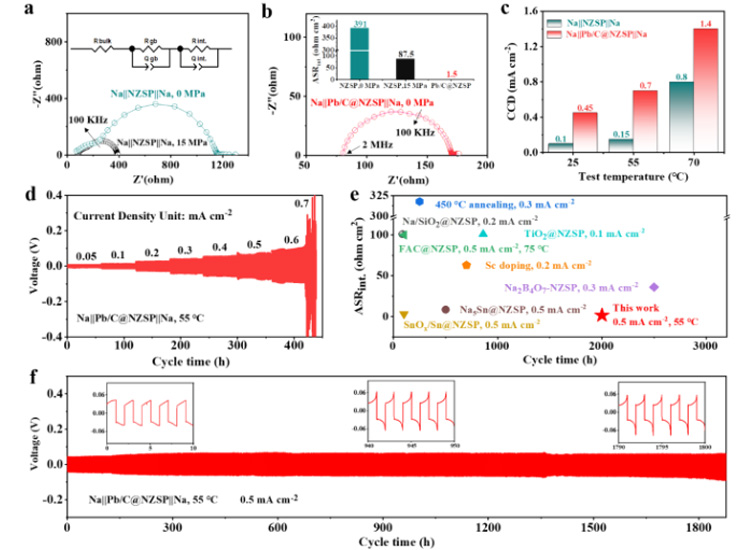
Figure 3. (a) 25 ℃, Na | | NZSP | | Na symmetric battery in 0 MPa and the impedance of the stack 15 MPa pressure diagram. (b) 25 ℃, Na | | @ NZSP Pb/C | | Na the impedance of the symmetric battery under 0 MPa pressure diagram. (c) Na | | NZSP | | Na and Na | | @ NZSP Pb/c | | Na symmetry battery at 25 ℃ and 55 ℃, 70 ℃ under the limiting current density (CCD). (d) and 55 ℃, Na | | @ NZSP Pb/C | | Na symmetric cell within the range of 0.05 0.7 mA cm - 2 deposit/stripping cycle curve. (e) Comparison of Pb/C@NZSP and other NZSP-based solid electrolytes for symmetrical batteries. (f) and 55 ℃, Na | | @ NZSP Pb/C | | Na symmetric cell cycle performance under mA 0.5 cm to 2.
4. Quantitative control of sodium negative electrode and performance of whole battery With perfect sodium wettability, sodium metal negative electrode was prepared in situ on the surface of Pb/C@NZSP solid electrolyte by molten sodium perfusion method. Due to the low resistance and dendrite-free negative interface, Pb/C@NZSP solid state batteries based on intercalated NVP and converted S positive electrode show better rate performance and cycle stability than unmodified full batteries (Figure 5a-b). The author points out that Na15Pb4 phase exist at the same time promote the diffusion of Na dynamics, thus delaying the Na | | @ NZSP Pb/C interface on the formation of porosity or empty.
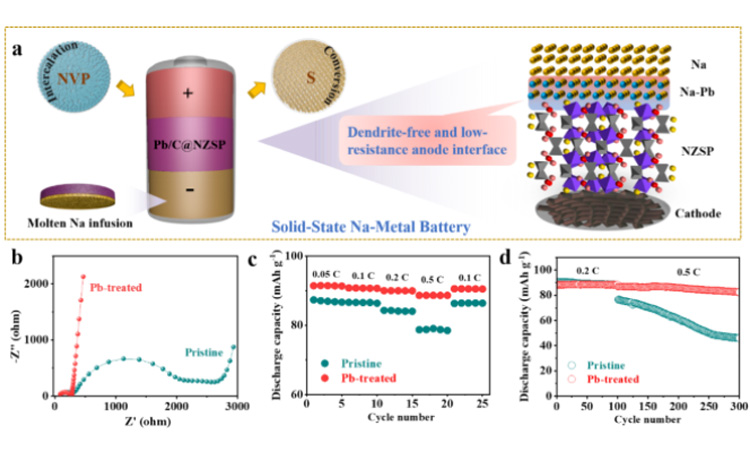
Na figure 4 (a) | | @ NZSP Pb/C | | NVP and Na | | @ NZSP Pb/C | | S solid-state batteries, highlighted the Pb/C interface engineering in homogeneous charge distribution and inhibit the growth of dendrite advantage. Na | | NZSP | | NVP and Na | | @ NZSP Pb/C | | NVP full battery (b) before the loop impedance spectroscopy and its ratio (C) and (d) long cycle performance.
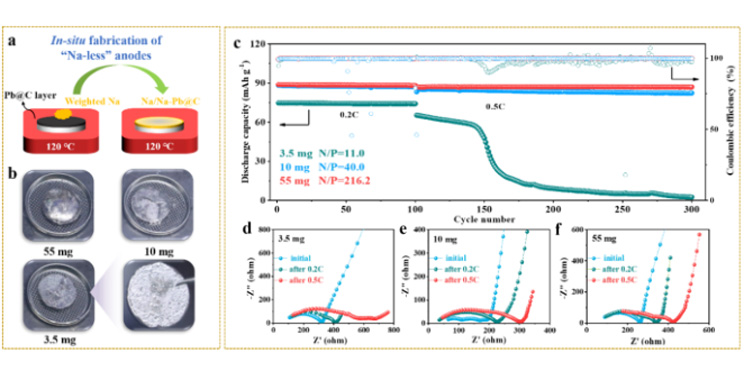
Figure 5. (a-b) In situ quantitative preparation of sodium cathode on Pb/C@NZSP surface. (c) Na | | @ NZSP Pb/c | | NVP full battery in 3.5 mg (N/P = 11.0), 10 mg (N/P = 40.0) and 55 mg sodium (N/P = 216.2) under the load of long cycle performance and (d - f) cycle impedance spectra.
To explore full battery performance at high sodium negative utilization (or low N/P ratio), we assembled solid sodium metal batteries by combining negative sodium electrodes of different qualities (3.5 mg, 10 mg, and 55 mg, respectively) with positive NVP electrodes. Both full cells with N/P ratios of 40.0 (10 mg) and 216.2 (55 mg) showed very stable cycle performance at 0.2C and 0.5C, with coulomb efficiency consistently exceeding 99.7%. However, the initial capacity of a full battery containing 3.5 mg Na (N/P=11.0) is only 75.0 mAh g-1. Although stable for 100 cycles at 0.2C, the discharge capacity drops sharply when the multiplier increases to 0.5C, accompanied by coulomb efficiency fluctuations that indicate a "soft short" (FIG. 5c). The evolution of EIS during the cycle (FIG. 5d-f) also shows the poor interfacial dynamics and stability of the 3.5mg battery. The surface and section of the negative electrode after cycling were analyzed by SEM (see original). Among them, the negative electrode surface of the 3.5mg battery was severely sodium poor after cycling, and sodium distribution was uneven in the direction of interface thickness. The authors note that a smaller sodium load (3.5 mg) cannot achieve full penetration of the Pb/C layer, resulting in insufficient active area for the charge transfer reaction. This dynamic disadvantage induces uneven sodium deposition behavior, which is more significant at high current density, and eventually leads to dendrite growth and soft short circuit. Although the strategy of further reducing the N/P ratio remains to be explored, considering that most solid sodium metal batteries in the literature use unlimited thick sodium tablets, the cyclic stability shown in this work under N/P of 40 and multiplier of 0.5C still shows the effectiveness of the dual-function interlayer design strategy.
Contact: Jason Wang
Phone: 13580725992
E-mail: sales@aooser.com
Whatsapp:13580725992
Add: No.429 Guangming Road, Shenzhen City, Guangdong Province
We chat
